- New business unit, named LARC Robotics, will simplify percutaneous renal access in Percutaneous Nephrolithotomy (PCNL) procedures, a minimally invasive surgical technique for managing kidney stones;
- First surgeries were already performed at the Landeskrankenhaus Hall Hospital in Austria, led by Dr. Udo Nagele, and the company will spend the coming months validating technical and clinical claims in both the US and Europe.
Austria-based Interventional Systems has decided to spin-off its validated technology for interventional radiology and oncology into the endourology space.
The new venture, LARC Robotics, will use Interventional Systems’ products, know-how, and years of expertise in the complex interventional radiology landscape to revolutionize the kidney stone market. More than a surgical tool, LARC Robotics is developing a full-fledged digital surgery solution that addresses common challenges.
“We realized that, despite its superior stone clearance rates when compared to other techniques, PCNL adoption was still low”, Interventional Systems’ CEO Pedro Costa explains. “This is because obtaining adequate renal access is not a trivial task and it relies greatly on the experience of the urologist performing the procedure. Adequate pre-operative planning and intra-operative robotic assistance are sure to help mitigate these problems”, Costa adds.
“After understanding the market need, the solution became clearer”, Costa continues: “Our robot simplifies gaining renal access as it allows for targeting under real-time fluoroscopy, and it will be offered in combination with a digital platform that will allow surgeons to visualize patient-specific, high-fidelity 3D reconstructions of the diagnostic scans to prepare for the procedure. The final component is a data and analytics module for post-procedural analysis, to find areas of opportunity and performance trends”, Costa further develops.
“Our platform is poised to help many urologists expand their practice and start offering PCNL treatments. The shift to ambulatory surgery centers has already happened, and there is a clear opportunity for wide-scale adoption of a flexible, small-footprint surgical robot that does not require workflow adaptations at a more cost-effective price point than other urology robots currently on the market ”, adds Costa.
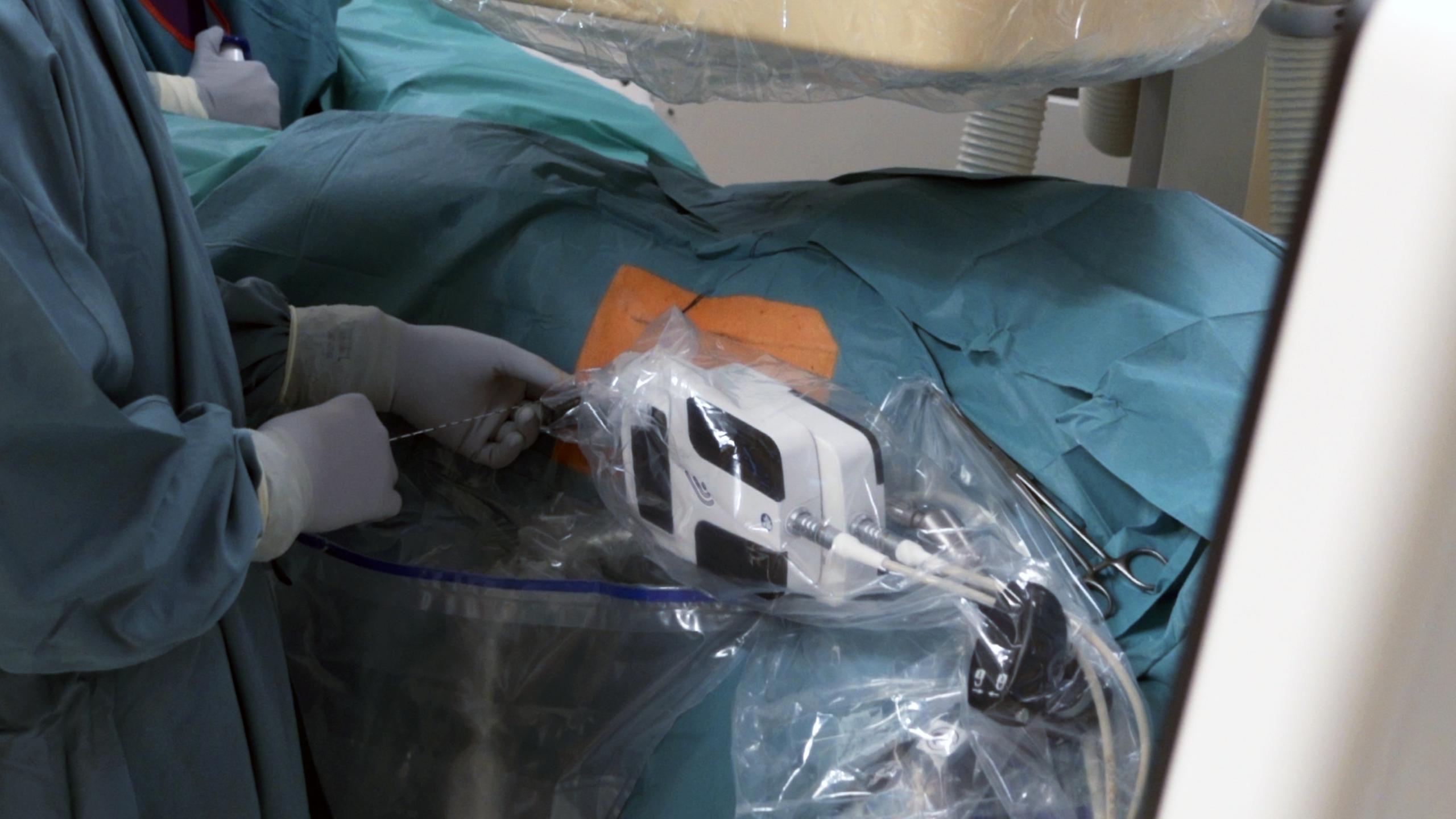
With clinical validation already underway, the company’s leader says “Everything went as expected with the surgeries that we already performed. It was “plug-and-play” and the benefits of robotic-assisted targeting were evident. We’re working with Dr. Nagele’s team, one of the most seasoned and high-functioning teams I’ve ever had the chance to witness, and it is a pleasure and a source of great learnings to continue developing the platform.”
The new technology is already CE-marked and FDA-cleared for fluoroscopy-guided procedures, and development has already begun for supporting ultrasound guidance as well.
“We know exactly what is coming. The technology is already in place, feasibility is finished, and we are focused on ensuring the entire market can adopt our robot without compromising their preferred equipment or imaging device,” says Srđan Milosavljevic, CTO. “Our main focus now is empowering urologists with a tool to gain appropriate percutaneous access, with fluoroscopy, ultrasound or both. We expect ultrasound compatibility to be available during 2024, and we’ll work from there towards incrementally resolving other challenges in the workflow.”
LARC Robotics is based off of New York, and its products will be first available for public demos at the American Urology Association Annual Meeting in the coming month of April.